lewis structure of naf|NaF Lewis Structure& Characteristics: 1 : iloilo This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, .
Hunter OneSearch lets you search in one place for: CUNY library catalog; a massive index of articles from journals, magazines, and newspapers; and unique digital content from the library. . Room Reservation. Reserve a study room or faculty office. Google Scholar. Expand your research results. Today's Hours. Leon & Toby Cooperman. 11:00am .Casino Royale (bra: 007 - Cassino Royale; prt: 007 - Casino Royale) é um filme britano-estadunidense de 2006, dirigido pelo neozelandês Martin Campbell, baseado no livro homônimo de Ian Fleming.. Trata-se do 21.º filme da franquia cinematográfica de James Bond e o primeiro com Daniel Craig no papel do agente 007; também é a terceira .
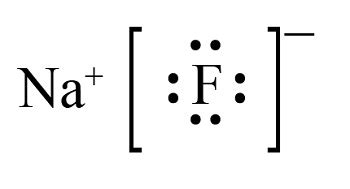
lewis structure of naf,A step-by-step explanation of how to draw the NaF Lewis Dot Structure.For NaF we have an ionic compound and we need to take that into account when we draw th. Subscribed. 205. 17K views 3 years ago Lewis Structures. Sodium, a metal in Group 1, loses one electron to become a +1 ion Fluorine, a non-metal in Group 17, gains one electron to .
Let us discuss about NaF Lewis structure and its 17 facts. NaF or Sodium fluoride is formed when the metal sodium reacts with halogen fluorine. It is odorless . The compounds in which the metals donate their electrons to the nonmetals have ionic bonds. .more. Sodium fluoride comprises one sodium and one flourine atom. As sodium and flourine atoms have .Sodium Fluoride | NaF or FNa | CID 5235 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, .
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, .sodium fluoride. Formula: FNa. Molecular weight: 41.9881725. IUPAC Standard InChI:InChI=1S/FH.Na/h1H;/q;+1/p-1 Copy. IUPAC Standard . Chemistry Questions Answered. Lewis Structure of Sodium Fluoride (NaF) [ionic] 8.3.2024. Sodium is a metal in Group 1 of the periodic table, and therefore brings .lewis structure of nafsodium fluoride. Formula: FNa. Molecular weight: 41.9881725. IUPAC Standard InChI:InChI=1S/FH.Na/h1H;/q;+1/p-1 Copy. IUPAC Standard .In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. Lewis Symbols We use .Lewis Dot Structure: Lewis dot structure is determined by the valence electrons of each atom therefore bond pairs/lone pairs represent the valence electrons. Among different types of structures, it is the best way which shows the charges, bonded electrons and lone pair electrons. Answer and Explanation: 1 Here is a video of this lewis structure being drawn step-by-step for you: Published by ChemistNate on March 8, 2024. Share this: Twitter; Facebook; Email; WhatsApp; . Lewis Structure of Sodium Fluoride (NaF) [ionic] 8.3.2024. Sodium is a metal in Group 1 of the periodic table, and therefore brings 1 valence electron with each .
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of . Drawing Lewis Structure of NOF. Step 1: To draw the Lewis structure of NOF we first need to choose a central atom. As nitrogen is the least electronegative element amongst all the three atoms involved it is chosen as the central atom. The Oxygen and Fluorine atoms are placed on each side of the Nitrogen atom.
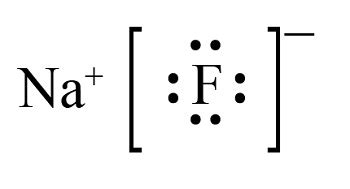
The Lewis structure, also known as the Lewis dot structure, is a visual representation of the valence electrons in an atom or molecule. It was developed by Gilbert N. Lewis in 1916 as a way to depict the bond ing and non-bonding electrons in a molecule. The Lewis structure is based on the octet rule, which states that atoms tend to gain, .Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3, you can decide shape of the NF 3 molecule. NF 3 lewis structure. In the lewis structure of NF 3, there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. Each fluorine atom has three .
lewis structure of naf NaF Lewis Structure& Characteristics: 1 Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, . Sodium fluoride comprises one sodium and one flourine atom. As sodium and flourine atoms have an ionic bond, it is also known as an ionic compound. The compo.
Lewis Structure Finder. Added Jun 9, 2014 by Tester in Chemistry. This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.NaF Lewis Structure& Characteristics: 1It is a dry chemical used in fluoridation of drinking water, it should be manually weighed and added to the mixing tank. NaF. Sodium fluoride. Density. 2.56 g/cm³. Molecular Weight/ Molar Mass. 41.98817 g/mol. Boiling Point. 1,695 °C.When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For example, consider the ammonium .Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms. (a) the amino acid serine: (b) urea: (c) pyruvic acid: (d) uracil: (e) carbonic acid: A . Lewis Structure of Lithium Fluoride (LiF) [ionic] 8.3.2024. Lithium is a metal in Group 1 of the periodic table, and therefore brings 1 valence electron with each atom: Fluorine is a non-metal in group 17 of the periodic table, and therefore brings 7 valence electrons with each atom: Fluorine requires ONE extra electron to complete its octet .
Table 4.5.2 4.5. 2: Lewis Dot Symbols for the Elements in Period 2. Ionic compounds are produced when a metal bonds with a nonmetal. Stability is achieved for both atoms once the transfer of electrons has occurred. The image below shows how sodium and chlorine bond to form the compound sodium chloride. Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the .Lewis structure of a water molecule. Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any .
Key to this theory is the Lewis Structure, which is a very simplified representation of the electrons in a molecule and is use to show how the electrons are arranged around individual atoms in a molecule. Between 1916 and 1919, Gilbert Newton Lewis, Walther Kossel, and Irving Langmuir formulated a theory to explain chemical .To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO 4 – 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button. Then we write the rest of the formula being .
lewis structure of naf|NaF Lewis Structure& Characteristics: 1
PH0 · sodium fluoride
PH1 · The Lewis structure in the naf theory
PH2 · Sodium Fluoride
PH3 · NaF Lewis Structure& Characteristics: 19 Complete Facts
PH4 · NaF Lewis Structure& Characteristics: 19 Complete Facts
PH5 · NaF Lewis Structure& Characteristics: 1
PH6 · NaF Lewis Structure
PH7 · Lewis Structure of Sodium Fluoride (NaF) [ionic] – ChemistNate
PH8 · Lewis Structure Finder
PH9 · How to Draw the Lewis Dot Structure for NaF:
PH10 · Draw the Lewis Structure of NaF (sodium flouride)
PH11 · 7.3 Lewis Symbols and Structures